In the 21st century, infectious viral diseases have become a problem on a global scale. Continued globalization has increased the speed at which viruses spread. The SARS-CoV-2 pandemic highlighted the need for effective and rapidly available antiviral compounds.
While more than 220 viruses are known to infect humans, clinically approved antiviral drugs are available for only a dozen of these viruses. Isolated, pure compounds from natural sources have the potential to provide solutions to global health concerns. In fact, many current medications have been derived from plants, fungi, and bacteria, and there is a critical need for additional antiviral agents to combat current and future viral outbreaks.
Centrifugal Partition Chromatography (CPC) is an ideal solution for natural compound investigations. Gilson, in collaboration with Brazilian researchers, recently performed preliminary experiments demonstrating the antiviral activity of compounds that were fractionated from a plant crude extract using CPC.
What is centrifugal partition chromatography?
CPC, also known as counter current chromatography, is a preparative, pilot- and industrial-scale liquid-liquid partition technique. Traditional liquid chromatography (LC) uses silica for the stationary phase, while CPC uses two immiscible liquid phases. The centrifugal field retains the stationary phase in the CPC rotor – equivalent to the column in traditional LC. CPC allows integral natural extracts fractionation as limited sample preparation is needed prior to injection and eliminate risks of irreversible adsorption of potential active compounds on the solid phase.
Chikungunya Virus
Scientists and pharmaceutical companies are urgently exploring plant compounds to find antiviral treatments, particularly for RNA viruses. The Chikungunya virus (CHIKV) is a good example of a virus that lacks treatment options. CHIKV is a mosquito-borne single-stranded RNA virus transmitted to humans by Aedes aegyptiand A. albopictusmosquitos, the same vectors responsible for the transmission of other viral diseases such as dengue, yellow fever, and Zika. Today, all those infectious diseases are considered emerging challenges to public health worldwide.
CHIKV was first isolated and described during an outbreak in southern Tanzania in 1952, after which it caused occasional outbreaks limited to Africa and Asia. Nevertheless, since 2004, the infamous Asian tiger mosquito has proved an excellent vector for CHIKV, causing the rapid spread of CHIKV throughout Asia, Africa, Europe, and the Americas. Chikungunya causes fever, fatigue, and severe debilitating joint pain. There is no vaccine for chikungunya, and treatments are limited to targeting specific symptoms such as fever and pain reducers.
Isolating Anti-CHIKV Compounds
The inhibitory effect of the compounds on CHIKV replication was assessed in a dose-dependent manner in cell culture. The cells were challenged with 50 PFU (plaque-forming units) of CHIKV and simultaneously treated with different fractionated extract concentrations ranging from 31.25 -125 μg/mL.Viral replication was assessed 48 hours post-infection by plaque formation assay.
Treatment of virus-infected cells during the entire post-inoculation period examined the antiviral effect of compounds during the latest steps, such as genome translation and replication, virion assembly, and virion release from the cells. The researchers’ findings demonstrated that adding the fractionated compounds after viral infection caused a relatively strong reduction (around 80%) in CHIKV replication.
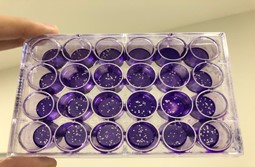
Figure 1. Plaque reduction assay made on a cell culture plate of 24 wells after liquid-liquid purification to evaluate potential antiviral activity. Each of the dots represent one virus particle. The inhibitory effect of the compounds on viral replication was assessed in a dose-dependent manner, with a constant challenge of 50 PFU per well.
Advantages of CPC for Antiviral Exploration
The CPC method is a rapid, single-step process that can be performed on a large scale to produce multiple kilograms of product with high purity, allowing for the cost-effective isolation of fractions from complex natural extracts. It offers advantages over small-scale purification methods, which may be inconsistent and have lower purity, complicating the early-stage research processes. CPC is also more environmentally friendly compared to other methods that may require large amounts of solvent.

Figure 2. VERITY CPC Pilot System, one of Gilson’s scalable CPC purification systems
View All CPC Systems
As the risk of global pandemics from current and future viral strains increases, the need for new antiviral compounds has become more urgent. The ideal antiviral compound can be generated quickly and be clinically effective against multiple strains of the same virus. The global scientific community is tackling this challenge with the help of CPC –a valuable technique to accelerate the identification of novel antiviral compounds from natural plant extracts. Plants contain a wealth of medicinal compounds, but partitioning is necessary to detect the compounds with the best antiviral activity to develop new pharmaceutical antiviral drugs.