Quantitative polymerase chain reaction (qPCR) is a powerful and highly sensitive method of amplifying genetic material, with applications ranging from diagnostics and forensics to research.
However, ensuring the accuracy and reliability of your qPCR experiments can be challenging — even the smallest amount of contamination or failure to adhere strictly to liquid handling best practices can substantially impact your results.
- Minimize contamination
qPCR's sensitivity is a great advantage, enabling scientists to detect the presence of even minute amounts of specific DNA sequences in a sample. But such sensitivity also leads to one of qPCR's greatest challenges — susceptibility to contamination. Just a single copy of an undesired DNA sequence can lead to false positives and render your research results unreliable. That could mean you need to rerun assays or entire experiments, adding to your research time and costs.
Contamination in qPCR has two primary sources: environmental contamination — any contamination introduced by experiment execution (producing aerosols when opening a tube, for example); and chemical contamination, whereby lab chemicals such as reagents, buffers, and nucleotides introduce contaminants into your qPCR reaction.
Thankfully, there are several things you can do to help reduce the risk of qPCR contamination:
- Use separate, dedicated spaces. Use dedicated spaces for different parts of your qPCR workflow, each with separate pipettes, tips, and personal protective equipment (PPE). At a minimum, you should segregate pre- and post-amplification areas of the lab, and the room's ventilation systems should be separate to avoid amplification carryover contamination.
- Decontaminate your surfaces and tools. A 10% bleach solution applied to work surfaces, equipment, and personal belongings (where appropriate) can help drastically reduce the risk of contamination in your qPCR workflows.
- Use task-appropriate tools. Poor choice of pipette and pipette tips can increase the chances of contamination in qPCR experiments. Selecting pipettes and pipette tips suitable for the sample type involved (e.g., positive displacement pipettes for viscous/volatile samples — more on pipette types later) and manufactured using high-quality materials is critical to keep contamination to a minimum.
For more details on the sources of qPCR contamination, how to detect it, and the key ways to reduce contamination risk, check out our dedicated blog on the topic.
- Normalize the amount of input DNA and RNA
Since the amount of nucleic acid can vary drastically between samples and that nucleic acid concentration can impact quantification cycles, it is important to quantitate and normalize input DNA and RNA concentration for your qPCR and RT-qPCR if you want to achieve accurate and reliable results.
Beware, though — normalizing nucleic acid concentrations can be error-prone since the process requires precise calculations and tedious, repetitive pipetting, with manual pipette adjustment needed at each process step.
- Use appropriate pipetting tools
Selecting the right pipetting tools and consumables and using them correctly can ensure your qPCR results are accurate and reliable.
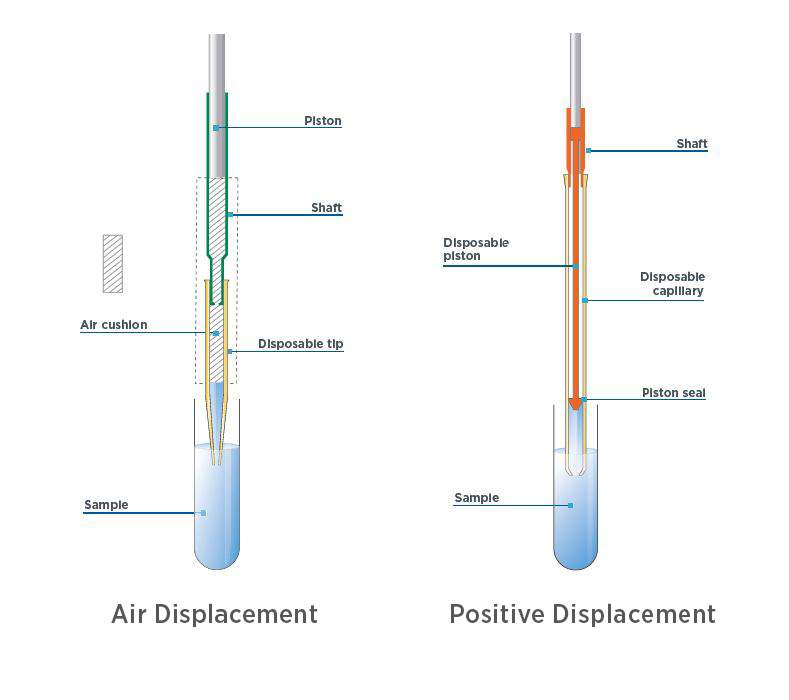
When selecting the best hand-held pipette for you, there are two main types: air displacement pipettes and positive-displacement pipettes (figure 1). The right choice depends on the type of liquids you'll be pipetting; if you're working with aqueous, non-viscous liquids, an air displacement pipette will be your tool of choice and can deliver high accuracy and precision (if used correctly)
Figure 1. Differences between a positive displacement and air displacement pipette
However, if your assays include viscous, volatile, hot/cold, foaming, or dense liquids, you'll want a positive displacement pipette. Unlike an air displacement pipette (which has an air cushion between the liquid and the piston), a positive displacement uses a disposable capillary piston tip where the piston comes into direct contact with the liquid you're pipetting. The lack of an air cushion means that the physical properties of the sample do not influence the volume delivery of the pipette, meaning this type of pipette delivers highly accurate and reproducible results, regardless of the liquid dispensed.
Similarly, the pipette tip you choose will influence the accuracy and precision of your measurements, too. There is a wealth of tip options to choose from, but it'll be hard to go wrong if you keep in mind the following:
- Air displacement or positive displacement. The two types of pipettes – air displacement and positive displacement, use different pipette tips to ensure accurate results. The former requires a standard tip, and the latter requires a capillary piston tip.
- Pipette tip fit. A poorly fitting pipette tip is one of the most common causes of inaccurate, imprecise, and unreliable pipetting. That’s why choosing a pipette tip that matches your given pipette is critical, enabling a firm, air-tight seal that prevents leakage.
- Pipette tip volume. Make sure that your tip matches the volume of your pipette and has a nominal (maximum) volume as close as possible to the volume of liquid you need to transfer. (The larger the difference, the less accurate your pipetting will be). Never cut pipette tips yourself, as this can lead to inaccurate and unreliable volume aspiration and dispensing.
- Filtered vs. unfiltered pipette tips. Filtered tips help minimize the risk of contamination during the aspiration and dispensing steps of pipetting by providing a barrier against aerosols and liquid splashes. Therefore, using filtered tips is a smart choice when working with assays that are highly sensitive to contamination (such as qPCR).
- Follow good pipetting practices
For accurate and reliable results, proper technique is just as important as appropriate tools and consumables. Thankfully, if researchers adopt best practices, they can drastically reduce the chances of error and variation in their pipetting, which means more accurate and reliable qPCR results.
First, always ensure your pipette tip is immersed at the right depth below the meniscus (3–5mm is often ideal), holding your pipette vertically during liquid aspiration and removal from the vessel.
It's also important not to rush during your pipetting. More specifically, you should pause for approximately one second (the exact time depends on properties such as the viscosity of your liquid) after aspirating and before you remove the pipette tip from the liquid. The liquid will continue to aspirate after the plunger stops, so prematurely removing the pipette leads to less sample aspiration than intended, which could compromise the accuracy and reliability of your results. Rushing can also lead to bubble formation in your aspirated liquid, or the liquid ‘jumping’, which can affect pipetting accuracy and reliability.
Finally, you should pre-wet your pipette tips when pipetting your sample and your master mix. Pre-wetting means aspirating and fully expelling a small amount of liquid/sample at least three times before aspirating for liquid transfer. Doing so will increase the humidity within the pipette tip, significantly reducing liquid evaporation and driving more accurate and reliable pipetting. What's more, immersing pipette tips can also partially limit carryover.
- Consider automation
Since many causes of error and unreliability in qPCR workflows stem from manual tasks, it's no surprise that automation can help unlock more accurate, precise, and reliable results. An automated liquid handling system, such as Gilson’s PIPETMAX®, can help with all of the above recommendations to drive more accurate and reliable qPCR results.
For instance, PIPETMAX can drastically reduce the risk of carryover contamination and cross-contamination by minimizing human intervention throughout liquid handling workflow steps. PIPETMAX can also automate the crucial error-prone RNA and DNA normalization process with the PIPETMAX Normalization Assistant. Using Normalization Assistant, sample volumes and required diluents are automatically calculated by the software (eliminating arithmetic errors), pipetting volumes are adjusted, and liquids are dispensed automatically. During these processes, sample information is easily imported and exported, preventing transcription errors and ensuring traceability.
Figure 2. PIPETMAX Automated Liquid Handling System
Additionally, by eliminating manual pipetting, tools can ensure pipetting is executed using optimal technique efficiently, time and time again. Additionally, deploying an automated solution for your qPCR has benefits beyond results accuracy and reliability. Automation solutions such as PIPETMAX can reduce the risk of repetitive strain injury (RSI) and increase qPCR speed and throughput by eliminating human interaction and repetitive manual tasks from qPCR assays. As a result, analysts benefit from better well-being and have more time to pursue value-adding work, and labs can realize greater productivity.
qPCR is a simple yet critical technique in the life sciences with a broad range of applications. But for the qPCR results to be valuable, they must be accurate and highly reliable.
Thankfully, this isn't difficult. You can drastically improve your qPCR results by following best practices for contamination reduction, normalizing your input RNA and DNA, and using the right liquid handling tools with the correct technique.
For labs that want to take their qPCR workflows to the next level — where they can more easily adhere to accuracy-enhancing best practices while unlocking a host of additional benefits — they should consider an advanced and fit-for-purpose automation solution such as the PIPETMAX®.
Want to learn more about how PIPETMAX could transform your qPCR workflows? Then check out our recent article, where we delve deeper into the system's unique capabilities.